Which Is the Most Basic Nitrogen in Each Compound.
I thought that the bond with more s-character increased in acidity but the answer is supposed to be 7. Up to 256 cash back Identify the most basic nitrogen atom on nicotine compound A and explain why.
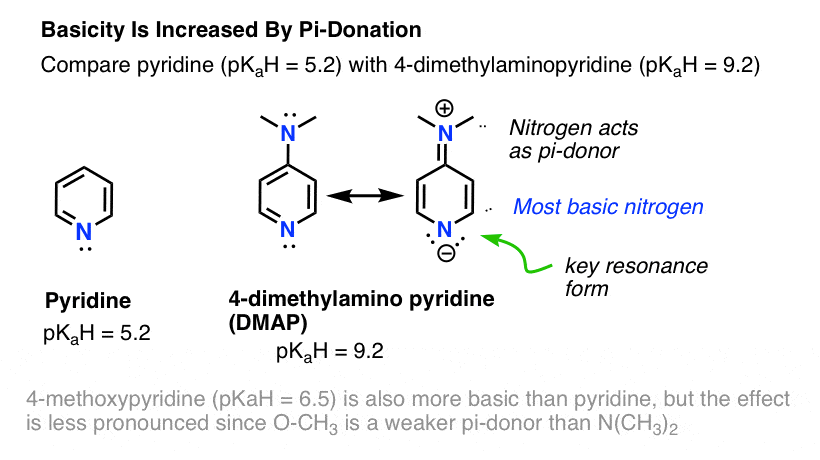
5 Key Basicity Trends Of Amines Master Organic Chemistry
Is the same true for the analogue of nicotine that is shown in compound B why.

. Experts are tested by Chegg as specialists in their subject area. Which nitrogen atom in the following compound is most basic. Imidazole pK a 695 is over a million times more basic than pyrrole because the sp 2 nitrogen that is part of one double bond is structurally similar to pyridine and has a.
A compound with molecular formula C6H15N exhibits a singlet at d 09 1H a triplet at d 110 3H a singlet at d 115 9H and a quartet at d 26 2H in its 1HNMR spectrum. 2021 Which is the most basic. Which of the following compounds has the most basic nitrogen.
Which nitrogen atom in each compound is more basic. The lone pair on b is more stable due to resonance than the lone pair on a. Guanidine H N C N H 2 2 IV.
The lone pair on nitrogen b is the most basic. Consider the structure of lysergic acid diethylamide LSD a potent hallucinogen containing three nitrogen atoms. Nitrogen is the chemical element with the symbol N and atomic number 7.
2021 Which is the most basic nitrogen in each compound. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table often called the pnictogens. 135-Triazine is the strongest base.
One of these three nitrogen atoms is significantly more basic than the other. Chemistry questions and answers. Since C has the least s character and no delocalisation so it is the most basic.
Which of the following is the correct order of basic character. Involved in resonance as shown. Therefore b is the weaker base and a is.
The question asks which nitrogen is most basic out of 2 1 7 or 9. Hello students the question is the most basic nitrogen in the following compound its citizens the compound given to us now basic nitrogen the most basic will be the nitrogen which can easily. Chemical Reactions of Amines.
From this we conclude that the most likely position to be protonated is N1 followed closely by N7 and also N3. Aniline 3 Aniline has a lone pair of electrons but they are partially involved in resonance delocalization into the aromatic ring. We review their content and use your feedback to keep the quality high.
Ammonia is a compound containing nitrogen and hydrogen atoms. Which is the most basic nitrogen in. The most basic nitrogen in the following compound is.
Lone pair on nitrogen 1 and nitrogen 2 are delocalised ie. The lone pair on nitrogen is delocallized and not fully available for donation making it less basic. The correct option is C Nitrogen 3 is the most basic.
More the delocalisation less is the basicity. Who are the experts. Off the charts is as expected N9 as it breaks the.
The most basic nitrogen in the following compound is A. Its chemical formula is rm N rm H_3 Ammonia is a covalent compound that is formed by the. Referring to the molecule above I know that pyridine C 6 H 5 N is a stronger base than water which I consider a type of alcohol so N is the most basic atom in this molecule.
The most basic nitrogen in the following compound is - Sarthaks eConnect Largest Online Education Community.
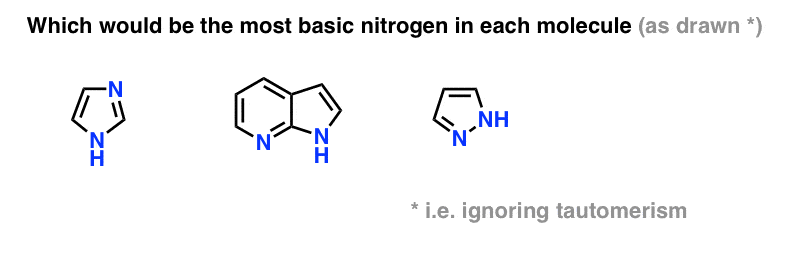
5 Key Basicity Trends Of Amines Master Organic Chemistry

Nitrogen An Overview Sciencedirect Topics

No comments for "Which Is the Most Basic Nitrogen in Each Compound."
Post a Comment